A Paper from FAHWMU Published on Nature for the First Time
 2023-02-08
2023-02-08
 Office of Cooperation and Communication
Office of Cooperation and Communication
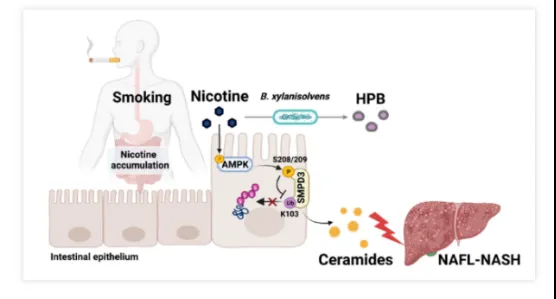
On October 19, Jiang Changtao's team from the School of Basic Medical Sciences, Peking University School of Medicine and Peking University Third Hospital Institute of Medical Innovation, together with Frank Gonzalez's team from the National Institutes of Health, Yu Zhaohui's team from the First Affiliated Hospital of Zhejiang University School of Medicine, Li Yang's team from the School of Basic Medical Sciences, Fudan University, and Zheng Minghua's team from the First Affiliated Hospital of Wenzhou Medical University (FAHWMU), published a research paper entitled "Gut bacteria alleviate smoking-related NASH by degrading gut nicotine" online in Nature. The research paper entitled "Gut bacteria alleviate smoking-related NASH by degrading gut nicotine" was published online in Nature, revealing for the first time that nicotine accumulates in the intestine during smoking and activates the intestinal epithelial AMPKα-neuramide axis. SMPD3-neuraminic acid axis, which promotes nonalcoholic steatohepatitis (NASH) through intestine-hepatic dialogue, and for the first time discovered that human intestinal flora can effectively degrade intestinal nicotine.
Zheng Minghua's team has been engaged in clinical and basic research on metabolism-related fatty liver disease for a long time, and has built a perfect clinical follow-up data and specimen database, which enjoys a high reputation in international and domestic. Up to now, he has co-edited the domestic "Expert recommendations for the standardization of the diagnosis and treatment of fatty liver disease in China (2019 revision)" (Chinese Journal of Liver Diseases 2019;27:748-753) and the international "New definition of metabolism-related fatty liver disease: an international expert consensus statement" (J Hepatol 2020;73:202-209) and "Clinical Practice Guidelines for Metabolic-Related Fatty Liver Disease of the Asia Pacific Society of Hepatology" (Hepatol Int 2020;14:889-919). The team is currently leading the development of 2 new international consensus on metabolic-related fatty liver disease.
In recent years, Zheng's fatty liver team has published/co-published a series of original works: (1) the first discovery that white adipose tissue-derived Sparcl1 can promote the inflammatory progression of NAFLD by regulating the expression of hepatic CCL2, a key molecular target for the regulation of the fat-liver axis, providing a new idea for the treatment of fatty liver (J Clin Invest 2021;131 :e144801); (2) confirmation that endogenous high-alcohol-producing Klebsiella pneumoniae strains can cause NAFLD (Cell Metab 2019;30:675-688); (3) N(6)-methyladenosine reading protein Ythdc2 inhibits hepatic steatosis by regulating the stability of lipid-producing gene mRNA (Hepatology 2021;73 :91-103); (4) myeloid-specific IL-6 signaling was found to promote MicroRNA-223-rich exosome production and attenuate NAFLD-associated fibrosis (Hepatology 2021;74:116-132); (5) in a clinical epidemiological perspective, the team constructed the largest Asian multicenter liver biopsy-based fatty liver cohort to explore and revise the value of the Baveno VI cut-off value in a population with progressive fibrosis in fatty liver, presenting Asian evidence for screening in high-risk populations (Aliment Pharmacol Ther 2021;54:470-480).


